Cs2 spell out the full name of the compound – Carbon disulfide (CS2), a versatile compound with a unique molecular structure, has garnered significant attention in various scientific and industrial domains. Its distinctive properties and wide-ranging applications have positioned it as a crucial component in diverse fields, shaping its historical significance and demanding a comprehensive exploration.
This article delves into the intricacies of carbon disulfide, unraveling its chemical composition, physical and chemical characteristics, and the multitude of industries it serves. Additionally, it sheds light on the environmental and health implications associated with its usage, underscoring the importance of responsible handling and safety considerations.
1. Explain the Compound Name CS2
Carbon disulfide (CS2) is a colorless, volatile liquid with a strong, unpleasant odor. It is a highly flammable and toxic substance. CS2 is used in a variety of industrial applications, including the production of rayon, cellophane, and other synthetic fibers.
The chemical structure of CS2 is a linear molecule with a central carbon atom bonded to two sulfur atoms. The carbon-sulfur bond is polar, with a partial positive charge on the carbon atom and a partial negative charge on the sulfur atoms.
CS2 is a nonpolar molecule, which means that it does not have a permanent dipole moment. However, the polar carbon-sulfur bond makes CS2 susceptible to attack by polar solvents, such as water and alcohol.
Properties of CS2
CS2 is a highly flammable liquid with a flash point of -30°C. It is also a toxic substance, with an LD50 of 100 mg/kg in rats. CS2 can cause irritation to the eyes, skin, and respiratory tract. Prolonged exposure to CS2 can lead to liver damage, kidney damage, and neurological problems.
2. Applications of CS2
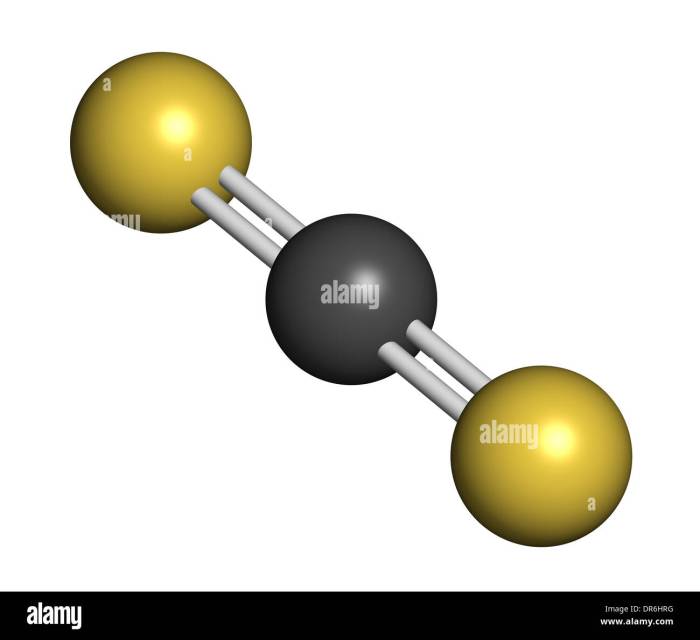
CS2 is used in a variety of industrial applications, including:
- The production of rayon, cellophane, and other synthetic fibers
- The manufacture of rubber and plastics
- The extraction of oils and fats from seeds
- The production of pesticides and herbicides
CS2 is also used as a solvent in a variety of chemical reactions. For example, CS2 is used to dissolve sulfur in the production of rubber. CS2 is also used to extract oils and fats from seeds. The oil and fat are then used to make food, soap, and other products.
Environmental and Health Implications of CS2 Usage, Cs2 spell out the full name of the compound
CS2 is a toxic substance that can have a negative impact on the environment and human health. CS2 is released into the environment through industrial emissions and the use of CS2-containing products. CS2 can contaminate air, water, and soil. CS2 can also bioaccumulate in the food chain, which means that it can be passed up the food chain from prey to predator.
Exposure to CS2 can cause a variety of health problems, including irritation to the eyes, skin, and respiratory tract. Prolonged exposure to CS2 can lead to liver damage, kidney damage, and neurological problems. CS2 is also a known carcinogen, which means that it can cause cancer.
3. Historical Significance of CS2: Cs2 Spell Out The Full Name Of The Compound
CS2 was first synthesized in 1813 by the German chemist Johann Wolfgang Döbereiner. Döbereiner prepared CS2 by reacting sulfur with charcoal. CS2 was first used commercially in the 1840s for the production of rayon. Rayon is a synthetic fiber that is made from cellulose.
Rayon is used to make a variety of products, including clothing, bedding, and upholstery.
In the early 1900s, CS2 was used in the production of cellophane. Cellophane is a transparent film that is made from cellulose. Cellophane is used to package food and other products. CS2 was also used in the production of rubber and plastics.
Today, CS2 is still used in a variety of industrial applications. However, the use of CS2 has declined in recent years due to concerns about its environmental and health impacts.
4. Safety Considerations for CS2
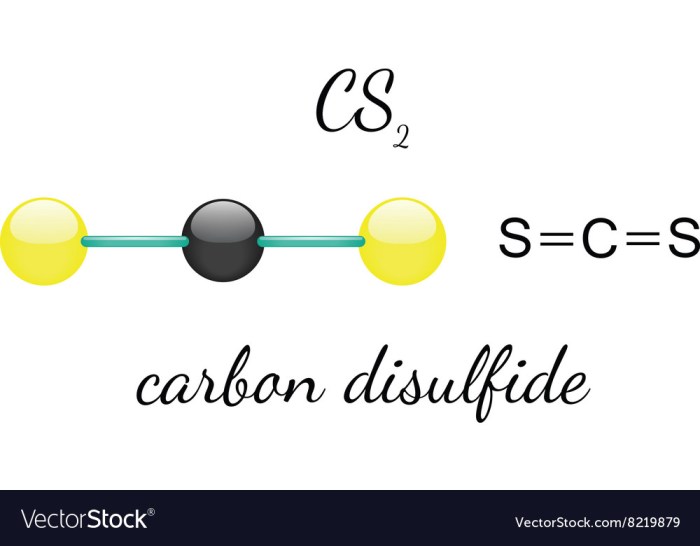
CS2 is a toxic substance that can be harmful if it is not handled properly. The following safety precautions should be taken when working with CS2:
- Wear appropriate personal protective equipment, including gloves, eye protection, and a respirator.
- Work in a well-ventilated area.
- Avoid contact with skin and eyes.
- Do not ingest CS2.
- Store CS2 in a cool, dry place away from sources of heat and ignition.
- Dispose of CS2 properly according to local regulations.
Clarifying Questions
What is the chemical structure of carbon disulfide?
Carbon disulfide (CS2) possesses a linear molecular structure consisting of a carbon atom covalently bonded to two sulfur atoms.
What are the physical properties of carbon disulfide?
CS2 exists as a colorless liquid at room temperature, exhibiting a pungent odor and high volatility. It is highly flammable and has a low boiling point.
What are the industrial applications of carbon disulfide?
CS2 finds extensive use in the production of rayon, cellophane, and carbon tetrachloride. It is also employed as a solvent in the rubber industry and as a fumigant in agriculture.